FDA approval means a product has been fully tested for safety and effectiveness. This includes lab research, clinical trials, and checks on manufacturing. Products that meet these standards gain FDA approval, allowing them to be used by the public. In this article, you can find the List of FDA-approved products & “not approved” products.
What does FDA Approved mean?
When the FDA grants approval to a drug, it signifies that CDER has thoroughly examined data regarding the drug’s effects. Furthermore, it concludes that the benefits it offers outweigh the known and potential risks for the specific target population.
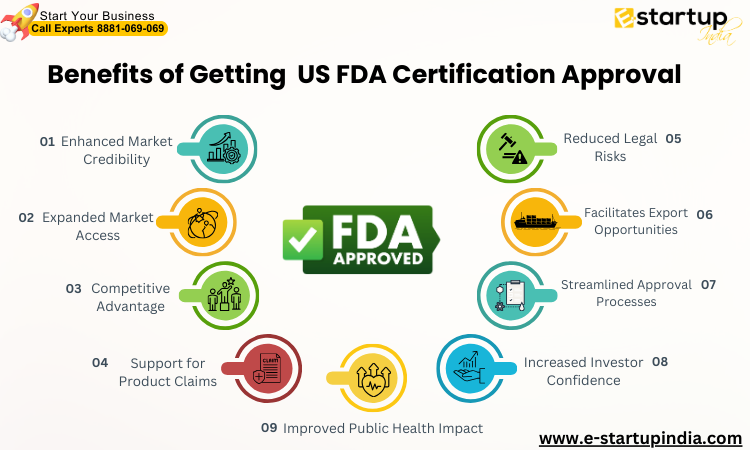
Benefits of getting US FDA Certification of Approval
-
Enhanced Market Credibility
US FDA Certification of approval lends credibility to your FDA product, assuring consumers that it has undergone rigorous testing and meets stringent safety standards. This builds trust and confidence in your brand, leading to increased sales and market share.
-
Expanded Market Access
FDA approval opens doors to broader markets. Many retailers, hospitals, and healthcare providers across the globe require US FDA Certification of approval before stocking or utilizing products, providing you with access to a wider customer base.
-
Competitive Advantage
Having FDA approval differentiates your product from competitors who may not have obtained the same level of regulatory clearance. This advantage can attract more customers and potentially command higher prices.
-
Support for Product Claims
FDA approval allows you to make specific claims about your product’s safety and effectiveness, which can be used in marketing materials and advertisements. These claims help you communicate the value and benefits of your product to consumers.
-
Reduced Legal Risks
FDA approval demonstrates that you have complied with regulatory requirements, reducing the risk of legal action and product liability issues. It provides a level of protection against lawsuits related to safety concerns.
-
Facilitates Export Opportunities
FDA approval through US FDA Certification in India is often a prerequisite for exporting products to international markets. Obtaining this approval can help you navigate global trade barriers and expand your business globally.
-
Streamlined Approval Processes
Once you have obtained FDA approval for a product, subsequent approvals for related products or variations can be expedited. This streamlined process can save time and resources for future product launches.
-
Increased Investor Confidence
FDA approval can instill confidence in potential investors, demonstrating that your product has met rigorous standards and has the potential for success in the marketplace. This can attract additional funding and support for business growth.
-
Improved Public Health Impact
US FDA Certification of approval ensures that your product is safe and effective, contributing to improving public health. Adhering to FDA regulations contributes to a safer and healthier society.
List of FDA-Approved Products
-
New Human Drugs & Biological Products
The FDA approves new drugs and biological products for human use. Before these products can be sold across state lines, they must receive US FDA Certification of approval, which requires demonstrating their safety, effectiveness, and adherence to quality standards.
FDA approval indicates that the benefits of the product outweigh the risks for its intended use. Biological products like therapeutic proteins, vaccines, and gene therapies require approval, and manufacturers must prove their ability to meet federal quality standards.
Rather than developing products, the FDA evaluates laboratory, animal, and human clinical testing conducted by manufacturers. The FDA also performs a lot of release testing to monitor product quality in real time.
-
Medical Devices
The FDA classifies medical devices into three risk-based classes: Class I, II, and III. Class III devices are the highest risk and require FDA approval through a premarket approval application.
Class II devices are at moderate risk and are generally cleared by the FDA for marketing if they are similar to legally marketed devices.
Class I devices are low-risk and are subject to general controls only.
| Class | Risk | Regulatory Requirements |
| Class I | Low | General Controls |
| Class II | Moderate | Special Controls or Premarket Notification 510(k) |
| Class III | High | Premarket Approval (PMA) |
-
Human Cells and Tissues
The FDA regulates HCT/Ps (human cells, tissues, and cellular and tissue-based products) to prevent infectious disease transmission. Furthermore, FDA approval is required for HCT/Ps with an elevated risk. However, The FDA does not regulate & approve the transplantation of vascularized human organs.
-
Food Additives in Food
The FDA does not approve food products, but it does approve certain ingredients before they are used in food. Companies must provide the FDA with safety information before adding new food additives. Approved food additives must be used in compliance with their approved uses, specifications, and restrictions. Certain food ingredients, such as those that are considered GRAS, do not require premarket approval by the FDA.
-
Color Additives in FDA Regulated
The FDA regulates the use of color additives in a variety of products, including food, dietary supplements, drugs, cosmetics, and some medical devices. Color additives (except coal-tar hair dyes) must be approved by the FDA before they can be marketed. The approval process includes an evaluation of safety data to ensure that the color additive is safe for its intended use.
List of FDA Not-Approved Products
-
Dietary Supplements & Other Foods
The FDA does not approve structure-function claims on dietary supplements or other foods. These claims must include a disclaimer stating that the claim has not been evaluated by the FDA and the product is not intended to diagnose, treat, cure, or prevent any disease.
-
Individual Food Labels
The FDA does not approve individual food labels, but it mandates specific labeling elements, such as nutrition information, on most foods and dietary supplements. Manufacturers must include serving size and nutrient content details on the “Nutrition Facts” or “Supplement Facts” label. Statements on food products must be truthful and comply with regulatory requirements.
-
Infant Formula
The FDA does not approve infant formula, but manufacturers must comply with federal nutrient requirements and other regulations. The FDA inspects facilities and collects product samples. If an adulterated or misbranded formula is found, the manufacturer must conduct a recall.
-
Medical Foods
Medical foods are specially formulated foods for people with specific dietary needs. They are not meal replacements or diet shakes, and the FDA does not approve it. However, Medical foods must comply with other regulations, such as current good manufacturing practices and registration of food facilities. They do not have to include a Nutrition Facts label, but any statements on their label or in other labeling must be truthful and not misleading.
-
Cosmetics
Cosmetics, such as perfumes, makeup, and shampoos, do not require FDA approval. The only exception is color additives (other than coal-tar hair dyes). Cosmetics must be safe for their intended use and properly labeled.
-
Tobacco Products
Tobacco products are regulated by the FDA based on a public health standard, not a safe and effective standard. Manufacturers must receive authorization from the FDA to sell or distribute new tobacco products in the U.S.
There are three pathways to market approval: premarket tobacco product applications, substantial equivalence applications, or exemption from substantial equivalence requests. Marketing authorization does not mean the product is safe or “approved,” but that the manufacturer has complied with the law.
-
Compounding Drugs
Compounding customizes medications for specific patients, but be aware that compounded drugs lack FDA approval, meaning they are not evaluated for safety, effectiveness, or quality.
Moreover, If you want any other guidance relating to the list of FDA-approved products & “not approved” products, Please feel free to talk to our business advisors at 8881-069-069.
Download E-Startup Mobile App and Never miss the latest updates narrating to your business.
